Guest Post by Janos Peti-Peterdi, M.D., Ph.D. and Georgina Gyarmati, M.D., Ph.D.
Our research team is driven by a personal and professional mission to find a cure for kidney disease, a growing global epidemic affecting one out of seven adults, which translates to 850 million people worldwide or about two million in the Los Angeles area. Kidney disease is one of the fastest growing global health problems associated with high individual, health care and societal costs, and high mortality. Currently, there is no cure for this silent disease. By the time kidney disease is diagnosed, the kidneys are irreversibly damaged and ultimately need replacement therapies. The only options are dialysis and transplantation.
As reported in our paper recently published in the Journal of Clinical Investigation, our team discovered a new mechanism by which the kidney tissue can repair and regenerate itself, and we demonstrated how targeting and augmenting this mechanism can be developed into a highly efficient regenerative therapy.
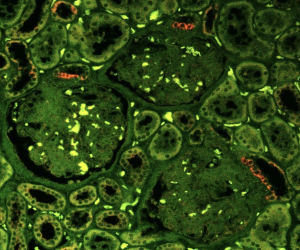
Our unique research approach initially focused on the healthy rather than diseased kidney, specifically on adaptive mechanisms to conserve body fluid and salt. Like the mechanical stimuli for bone repair and fasting for digestive system repair, the organ-specific physiological stimulus for kidney adaptation and repair is the loss of body fluid and salt. We mimicked this condition by reducing dietary salt intake and applied a powerful microscope and imaging technology to look inside the living kidney.
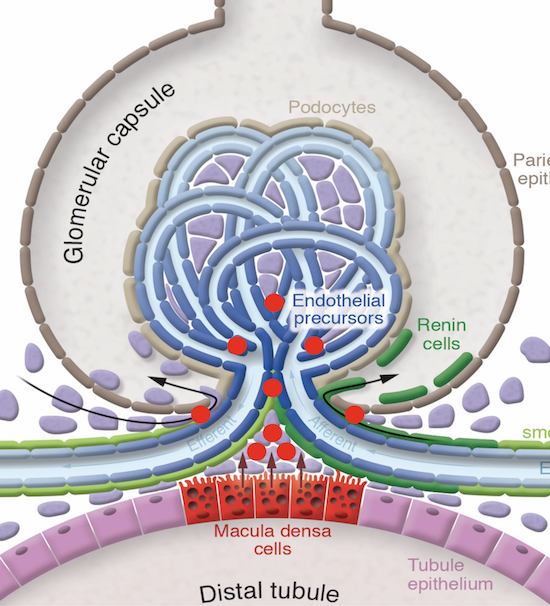
Using this approach, we uncovered the key role of macula densa cells in the repair process. The macula densa (MD) is a unique group or niche of a minority kidney cell type in each nephron, the building block of the kidney. The MD is formed by about 25 individual MD cells localized in a strategically central position at the vascular pole entrance of the kidney filter, the glomerulus. The MD is a main sensor in the kidney detecting alterations in the local and systemic environment including urine salt content and tissue metabolism, and based on this sensing information, MD cells signal to other kidney cells to regulate numerous kidney functions including glomerular filtration rate and kidney blood flow.
The true nature of MD cells has long been unknown due to their inaccessibility. However, this new study confirmed that MD cells are neuron-like cells, and via the release of many protein factors, they communicate with stem/progenitor cells to remodel and regenerate the kidney tissue. One such MD protein factor, CCN1 binds to molecules in the tissue matrix between cells and on the surface of target cells to promote the development of new blood vessels as well as to block the formation of tissue scars (so called fibrosis). Through subsequent work, we identified the specific genes and many other MD factors in addition to CCN1, which guide the growth, migration and transformation of stem/progenitor cells into many types of kidney cells, thus orchestrating highly efficient tissue regeneration. When we augmented these MD factors in a pre-clinical model of kidney disease using either genetic or pharmacological tools, we observed robust kidney tissue regeneration and improved organ function.
Given the successful human and therapeutic translational studies reported in this JCI paper, efforts are already ongoing to develop a new class of medicine called MD mimetics. These drugs aim to augment the kidney tissue regenerative functions of MD cells for the regenerative cure of chronic kidney disease. The primary patient group target of MD mimetics are CKD patients in the early-mid phase of disease (Phase 1-3, representing >95% of CKD patients, or almost 40 million people in the US) when the kidney still has capacity to remodel and regenerate itself. It is expected that most CKD patients, irrelevant to the primary cause of kidney disease would benefit from this new therapeutic approach, including patients with advanced diabetes, minimal change disease, thin basement membrane, membranous glomerulonephropathy, IgA nephropathy, lupus nephritis, vasculitis, hypertensive nephropathy, and focal segmental glomerulosclerosis (FSGS).
In the next phase of research, we aim to further improve our understanding of the function of MD cells, which will be key to developing successful tissue-regenerative therapeutic strategies for the kidney, and perhaps other organs as well.